iSAEC’s Historical Background
The International Serious Adverse Event Consortium (iSAEC) is a pharmaceutical industry and FDA led international consortium, focused on identifying and validating DNA-variants useful in predicting the risk of drug induced, rare serious adverse events [SAEs]. The SAEC was launched in 2007, with the scientific, technical and financial support of eight initial research funding members (i.e Abbott, GSK, J & J, Novartis, Pfizer, Roche, Sanofi-Aventis, and Wyeth). Additional dues-paying members were added as the consortium executed its Phase 1 research program and launched its Phase 2 research program, including the Wellcome Trust, Takeda and Daiichi Sankyo. In addition, the Wellcome Trust provided financial and management support to the iSAEC during its first phase (2007-2010). The FDA and other regulatory bodies responsible for drug and medical products safety have been involved in all facets of the iSAEC as “Associate Members”. The iSAEC collaborates with leading (academic and industrial) scientists to identify the optimal genomic and computational methods to apply whole-genome SNP mapping/sequencing technology to discover and replicate associated SAE genetic markers. As part of its commitment to enable and facilitate research in this important area, the iSAEC releases all “allowable” data (i.e. as specified in the informed consents and allowed by IRBs) to all qualified researchers via its web-based “SAE data portal”.
The impetus for the iSAEC arose from a series of interviews conducted in late 2005 and early 2006, with senior research and development leaders in major pharmaceutical companies and government. The goal of these interviews was to explore how to best leverage the success of previous private-public consortia (e.g. The SNP Consortium) to identify additional areas to apply this effective “collaborative model”. Out of ten possible collaborative opportunities, the highest priority was assigned to exploring the genetic and molecular basis of drug induced, rare serious adverse events. “Scale’ is critical in the practical realization of such a research focus, as no one company or organization is likely to have the clinical, patient, and genomic research resources to identify, screen and enroll adequate numbers of high quality subjects. Research on rare, drug induced SAEs is ideally suited to the “consortium model”.
Concurrent with the above mentioned market research, an FDA’s Industrial Advisory Board recommended an “independent industrial biomedical consortium” be formed to bring industry and regulators together to build a better understanding of the biology of drug induced serious events. Such a private sector led scientific collaboration was likely one of the initiatives the FDA had in mind when it developed and published its “The Critical Path: Accelerating the Development of Medical Products”. From July of 2006 to August of 2007 when the iSAEC was launched, Janet Woodcock, MD (director of the Center for Drug Evaluation and Research at the FDA) co-chaired the “Organizing Committee” of the SAEC with Arthur Holden, the founder and current chairman and CEO of the iSAEC.
Drug-induced, rare SAEs can be a severe health issue; and pose significant issues for the use of approved drugs and the development of new drugs. SAEs are a significant issue for patients, FDA, industry, and payors; and an important element contributing to healthcare cost inflation. Examples of drug-induced, rare SAEs include drug induced liver injury (DILI), drug induced kidney injury, QT prolongation, rhabdomyolosis, serious skin rashes (e.g. SJS), edema, acute renal failure, acute hypersensitivity, anemia, neutropenia, excessive weight gain, retinopathy, and severe vasculitis. The rarity of these drug induced SAEs and the absence of effective government surveillance/research networks, makes it extremely difficult for any one company/research entity to accrue enough SAE cases and controls to conduct effective whole genome studies. Central to the notion of the iSAEC is that industry, government and health care providers can join forces and work together to make use of a variety of sample and data resources to research the genetic basis of rare, drug induced SAEs.
Specifically, the iSAEC works to coordinate with existing SAE research networks, while fostering the development of new (novel) networks to obtain well phenotyped SAE cases and controls. The iSAEC collaborates with leading academic and industrial clinicians/scientists, HMOs and EMR providers to identify and enroll suitable “cases” and to apply optimal genomic and computational methods for SAE genetic marker discovery and validation. All “allowable” data (i.e. as specified in the informed consents and approved by the relevant IRBs) from these efforts will be released to qualified researchers free of cost.(See iSAEC Data Release and IP Policy)Organization of iSAEC
The iSAEC is a 501 c3 non-profit membership corporation (under the Internal Revenue Service Code) formed to engender the required organization, processes, and resources to identify and validate DNA-variants useful in predicting the risk of drug induced serious adverse events (SAEs). As such, it functions with the explicit purpose of enhancing the “public good”. Its members are (i) organizations engaged principally in the business of discovering, developing and marketing pharmaceutical products, or (ii) a charitable, governmental, or other non-profit organization with an interest in the field of medical science. To protect its members from the risk of frivolous law suits associated with such intra-industry sponsored research, the SAEC is registered under the National Cooperative Production and Research Act of 1993.
The purposes of the SAEC are:
- To carry out research directed toward the discovery of DNA-variants that are clinically useful in understanding and predicting the risk of drug induced serious adverse events and similar scientific research.
- To ensure the widespread availability of the results of such research to the scientific research community and the public at large for no charge through publication or other methods; and
- To educate the scientific research and medical communities about issues related to severe adverse drug reactions and about issues related to the Corporation’s research.
The iSAEC is governed by a Board of Directors (BOD) which has management control over the property, activities and funds of the Corporation. The BOD consists of one director from each Sponsoring Member and the Chief Executive Officer, ex officio. The Board structure also allows for involvement of other non-profit research and governmental organizations via “Associate Membership”. The iSAEC BOD functions and makes its decisions using a “majority rules” model. All key research collaborations are formed via a rigorous “RFP Process”, with final selection determined by the Scientific Management Committee. All sponsored research collaborations are governed by milestone based agreements, whereby the SAEC preserves its right to restructure or exit any collaboration where “underperformance” is a recurring issue. The iSAEC retains independent financial management and has the right to audited financial statements. The iSAEC also retains dedicated legal counsel.
The iSAEC BOD has appointed two major Committees to advise the SAEC on research and policy matters. The advisory committees are the Scientific Management Committee (SMC) and the Public Relations/Communications Advisory Committee. Exhibit 1 below summarizes the current organizational structure of the SAEC.Exhibit 1:
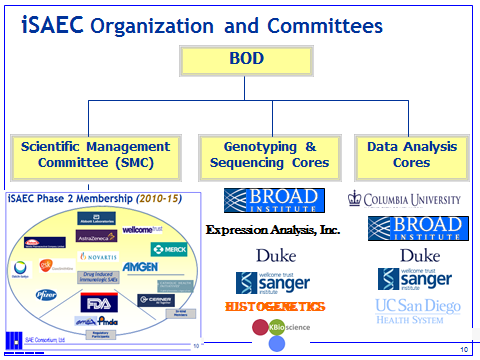
The SMC is co-chaired by Matt Nelson (GSK) and Sally John (Pfizer). Its charter is to provide the membership with sound research strategies, well defined research programs; including plans, milestones & performance monitoring and guidelines. It also provides advice on study design and methodology, phenotyping, clinical collaborators, CRF design, informed consent, genomic research matters, whole genome data analysis, and data release policies. The SMC facilitates iSAEC publications arising from its research activities, prior to public data release. The committee will encourage active participation of scientific and clinical experts from each of the SAE’s scientific collaborators. Each funding member will have a minimum of one representative on the SMC. Associate members, such as the FDA, have multiple members on the committee. In addition, the SMC invites adhoc (scientific or clinical) experts to consult on its research activities. Drs. Mark Daly (Harvard/Broad Institute) and Tom Urban (Duke) are currently two such SMC advisors.
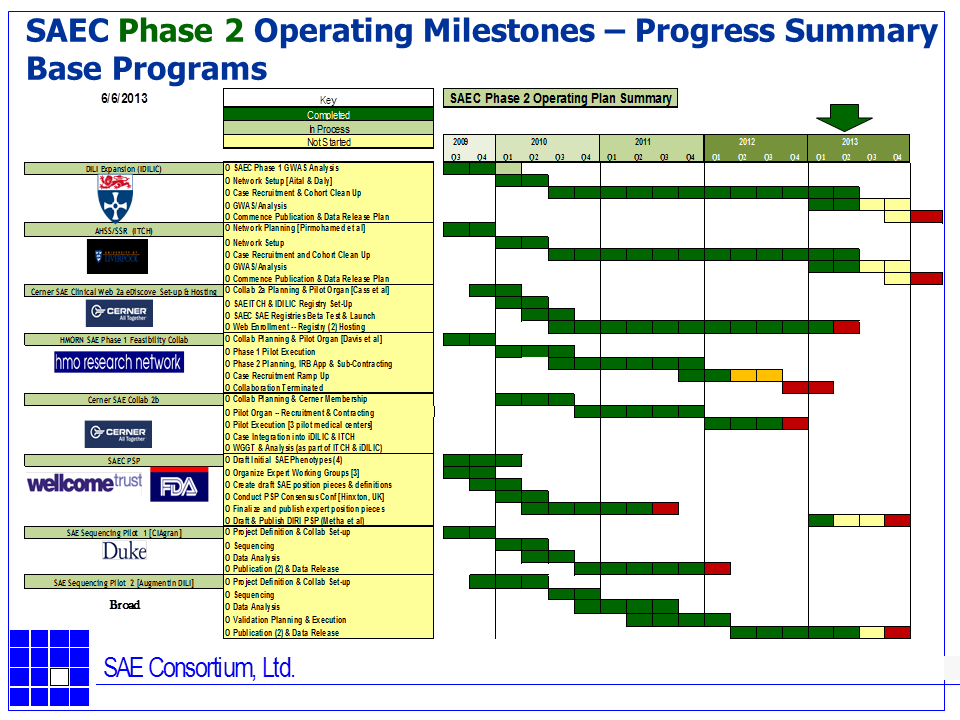
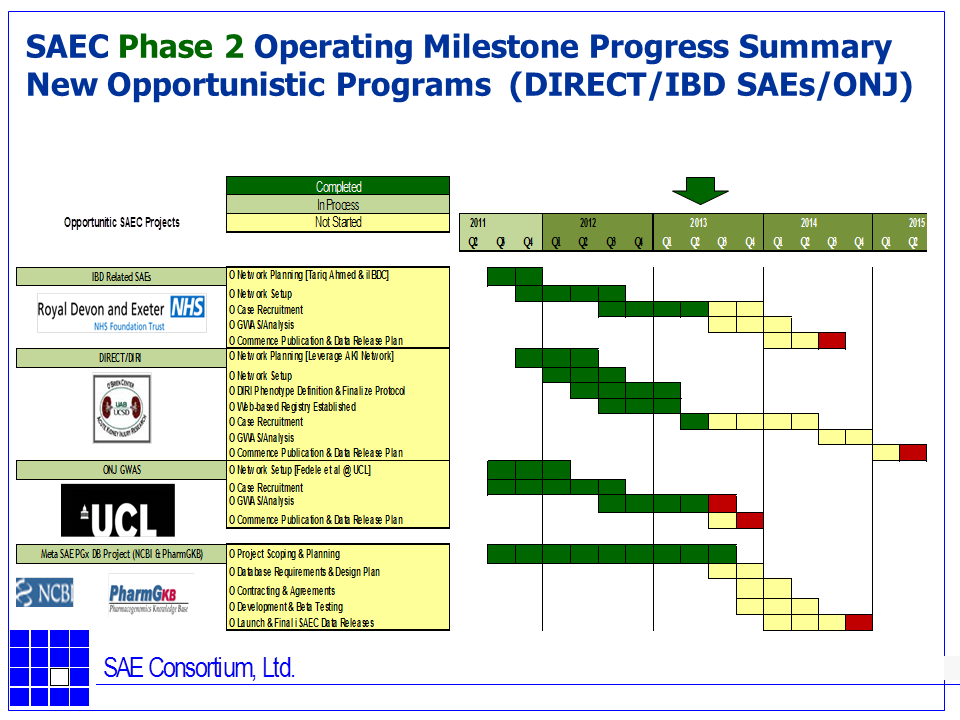
Graphical Operating Plan Summary and Status (as of June 2013) of the iSAEC
The following GANT charts summarize the iSAEC core Phase 2 projects and their current status of as June, 2013.