June 6, 2013
Dear Colleague and Friends:
Welcome to the website of the International Serious Adverse Event Consortium [iSAEC]. We are delighted you opted to spend a few minutes to understand our novel research enterprise.
We launched the iSAEC in August of 2007, as a novel international biomedical research consortium, focused on identifying and validating DNA-variants useful in understanding the risk of rare, drug induced serious adverse events [SAEs]. Drug-induced SAEs present significant health issues for certain patients, while posing significant challenges to the developers of new drugs and the users of approved drugs. The rarity of drug induced SAEs and the absence of effective government surveillance/research networks, makes it virtually impossible for anyone company/research entity to accrue enough SAE cases and (matched) controls to conduct genomic studies to illuminate underlying genetic risk factors. Interestingly, the (U.S.A.) National Institutes of Health has remained relatively inactive in this vital area of genomic research. Central to the notion of the iSAEC is industry, academic and health care researchers from around the world can join forces and work together; using state-of-the-art biological, clinical and genomic resources to effectively research the genetic basis of drug induced SAEs. This challenge demands a highly coordinated, well-resourced collaborative effort involving numerous international parties under the direction of experienced/dedicated leadership. It is my responsibility to provide this leadership.
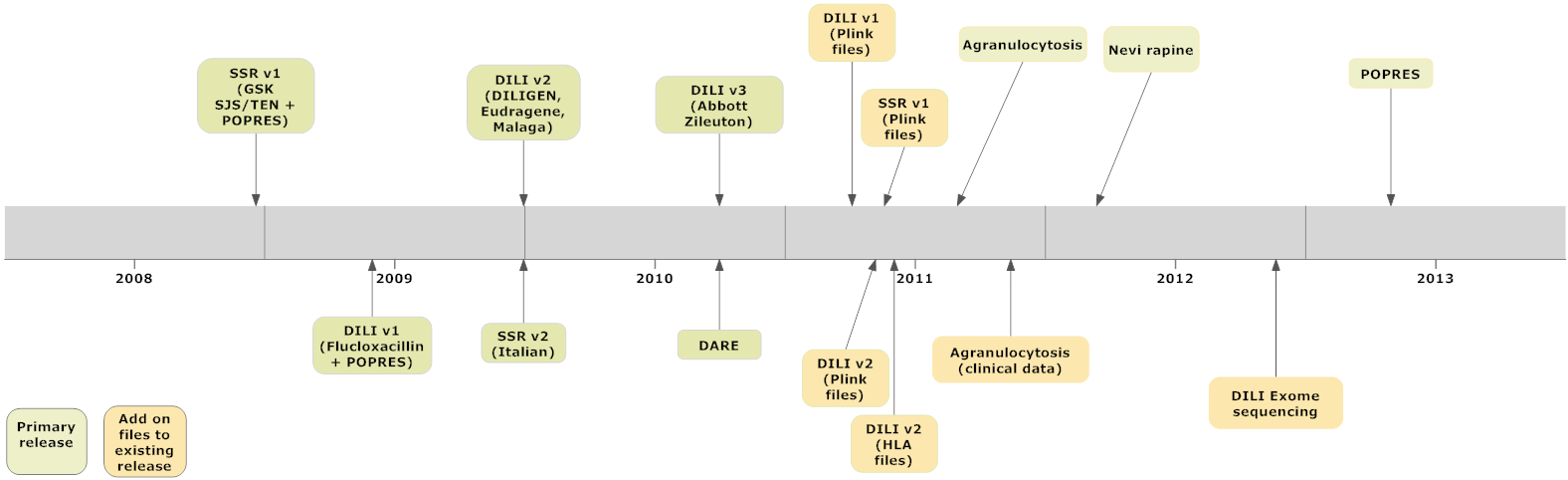
Although the Consortium’s scientific scope is broad in principle, it focused during its initial phase (2007-2010) on two core SAE research “pilot” projects. We collaborated with a variety of clinical and scientific partners to identify significant genetic variants associated with both drug-induced liver-disease [DILI] and serious skin injury [DISI]. The results and supporting data from these projects have/are being published in peer review journals. To date, we have approximately 10 significant scientific publications, with an equal number under development. Our research is significantly expanding both our knowledge, and the supporting primary clinical and genomic data publically available to qualified biomedical researchers interested in the genetics of drug safety. All “allowable” anonymous data generated from our research efforts is released to all qualified researchers at the same time. These raw data can be requested via this web site, by qualified researchers who sign a “data use agreement”. Figure 1, below, summarizes the iSAEC’s public data releases to date.
The iSAEC is helping to set a precedent for genetic analysis of drug-induced SAEs. Let me make a few points relative to our phase one findings:
- First, we have clearly demonstrated that “common” genetic variants, with a significant impact on the risk of serious adverse events, can be detected in relatively small sample sizes (<50 cases). This bodes well for the feasibility of applying genomic methods to such medical challenges. With good collaboration, we can aggregate cohorts of this size in a reasonable period of time.
- Second, most of our findings to date have been specific to a drug versus across multiple drugs.
- Third, our findings clearly demonstrate an important role for the MHC genomic region in the pathology of immunologically mediated SAEs such as DILI and SSR. Our research findings also emphasize the importance of immune regulation genes, in addition to a number of well characterized ADME genes.
- Lastly, a number of cross drug/ SAE genetic alleles are starting to emerge that may provide important insights into the underlying biology/mechanism of SEAs (e.g. HLA*5701 or UGT1A1*28).
These findings have helped us shape the second phase of the iSAEC (2010-2015). We have developed novel international research consortia and new partnership with large US healthcare systems to aggregate targeted case cohorts, associated with specific SAEs and causal drugs. Through our clinical networks we are deepening our understanding of the genetics of following drug induced SAEs:
- Hepatotoxicity (DILI)
- Serious Skin Rash (DISI)
- Acute Hypersensitivity Syndrome (DRESS)
- Nephrotoxicity (DIRI)
- TdP/PQT effects (DITdP)
- Excessive Weight Gain (AP cl2)
- IBD Therapy Related SAEs (4), and
- Jaw Osteonecrosis (ONJ)
We continue to apply novel state-of-the-art genomic methodologies to fulfill our research aims. In addition to whole genome genotyping, we are piloting the application of next generation sequencing to explore the role “rare” genetic variants in SAEs. We will also conduct biological pathway analysis, using specialized assays to better understand the underlying biology.
The iSAEC conducts its work with the scientific, technical and financial support of its pharmaceutical/other members. We are deeply indebted to Abbott, Diiachi-Sankyo, GSK, J & J, Novartis, Pfizer, Roche, Sanofi-Aventis, Takeda, the Wellcome Trust, and Wyeth for their enabling support of the Consortium’s phase 1 research. We also appreciate the additional support of our phase 2 research efforts being provided by Amgen and Merck. In phase two, we have also brought on board two "in-kind" members (Cerner, Inc and Catholic Health Initiatives) who are have made vital contributions to our phase 2 research. Finally, we are grateful for the collaborative spirit, team work and support provided to the iSAEC by the US FDA, Japanese PMDA, and the European EMEA.
Sincerely,
Arthur L. Holden
Chairman and CEO
International SAE Consortium, Ltd.
| Figure 1 iSAEC Data Release Chronology (2008-13) |
 |
